20 MIN READ
Corn Nutrition 101
August 19, 2024
It is important to understand the principles of soil fertility to efficiently manage corn nutrients, grain production, and environmental stewardship. Seventeen chemical elements are known to be essential for plant growth, and 14 of them are supplied by the soil. Each essential nutrient is needed in various amounts by the plant, differs in concentration in the components of a mature corn plant, and varies in mobility within the plant. Knowing the relative amount of each nutrient removed at harvest is useful for calculating the amount of fertilizer to apply to help maintain yield potential for subsequent crops.
Essential Nutrients
To be classified as “essential,” a chemical element needs to meet the following criteria:
- The plant cannot complete its life cycle (seed to new seed) without the element.
- The elements’ function cannot be replaced by another element.
- The element is directly involved in the plant’s growth and reproduction.1
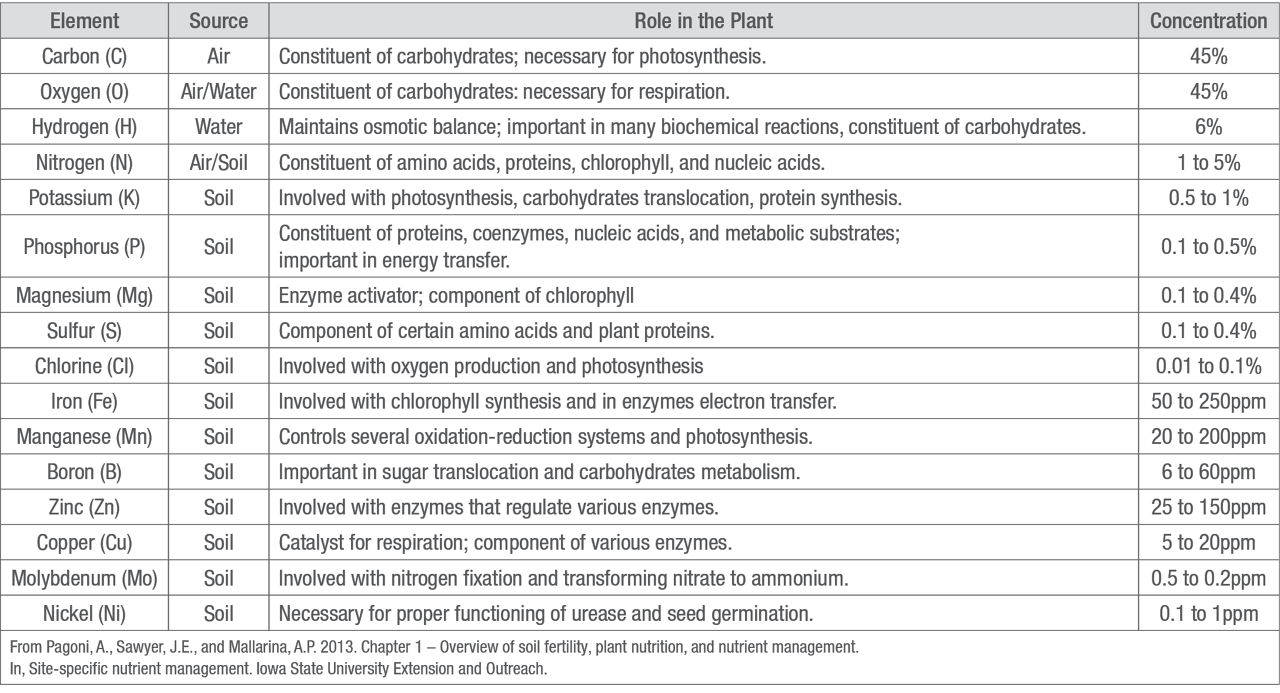
Table 1. Essential plant elements, source, roles, and relative quantities in the plant.
Non-Mineral Nutrients
Plants acquire non-mineral nutrients from water and/or air instead of from soil. Three elements are essential non-mineral nutrients for corn plants: carbon (C), hydrogen (H), and oxygen (O). Although these nutrients make up approximately 95% of plant biomass, they are given little attention in plant nutrition because they are usually in sufficient supply. However, other factors such as soil management and environmental conditions can influence the availability of these nutrients and a crop’s growth response to them.1

Mineral Nutrients
The 14 essential mineral nutrients are classified as either macronutrients or micronutrients based upon plant requirements and relative fertilization needs.1
The six macronutrients are nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and sulfur (S). The macronutrients N, P, and K are often classified as primary macronutrients, because deficiencies of N, P, and K are more common than deficiencies of the secondary macronutrients, Ca, Mg, and S. Each macronutrient accounts for 0.1 to 5% or 1,000 to 50,000 parts per million (ppm) of the dry weight of corn plant tissue.1
In comparison, each micronutrient generally comprises less than 0.025% or 250 ppm of dry plant matter (Table 1). The eight micronutrients are boron (B), chlorine (Cl), copper (Cu), iron (Fe), manganese (Mn), molybdenum (Mo), nickel (Ni), and zinc (Zn).1
How Plants Take Up Nutrients
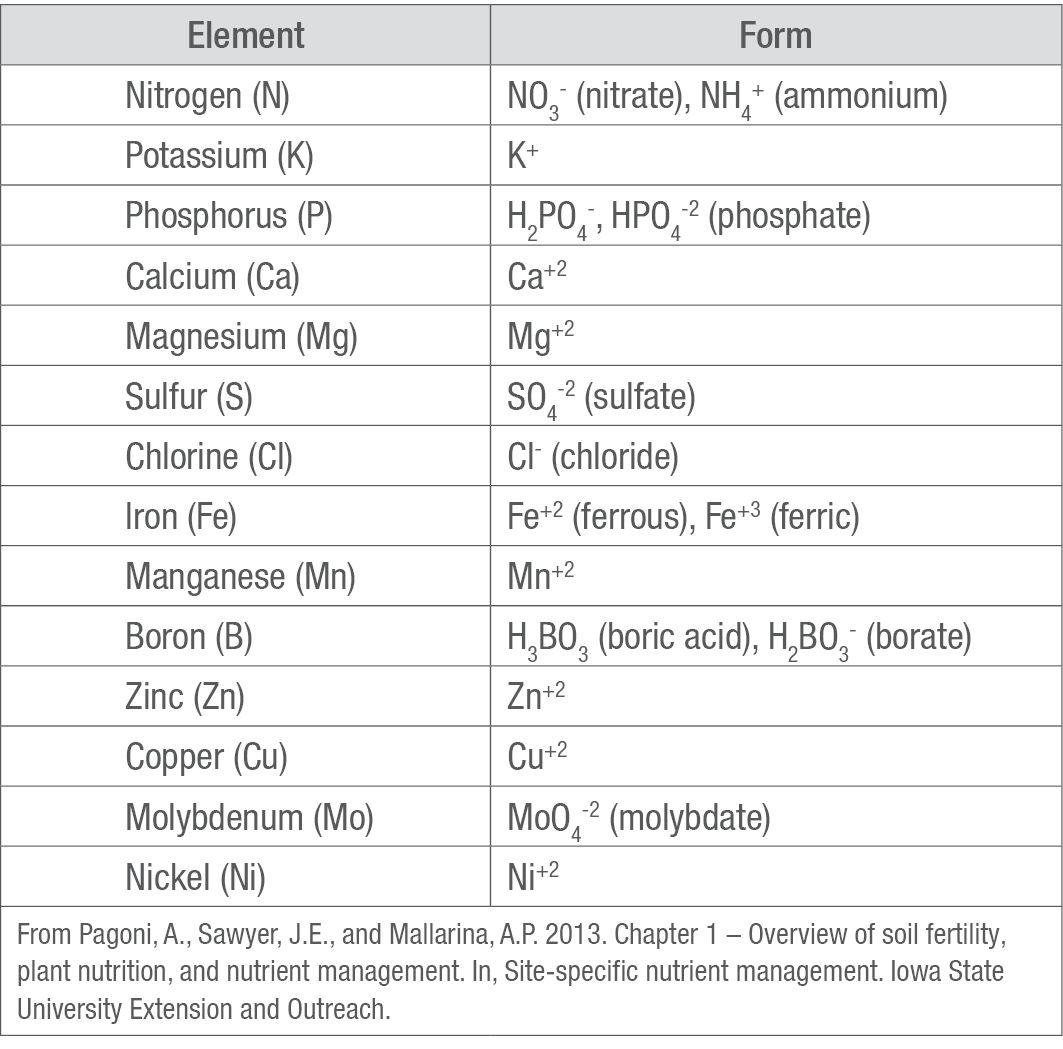
Chemical elements like essential nutrients exist in the environment in different chemical forms, but not all forms can be absorbed by plants. For example, most nutrients must be in an ionic (charged) form to be absorbed by a plant, except boron (B) which can be absorbed in the form of uncharged boric acid (Table 2).1 Most fertilizer products are combinations of the available forms of nutrients, so that the nutrients in the fertilizer are available to plants immediately or soon after application. A plant’s ability to absorb chemically available nutrients is determined by the size of its root mass (number of roots, root dry matter, and root length), the roots’ ability to absorb nutrients, and the concentration of available nutrients at the surface of the roots.1
Table 2. Nutrient forms taken up by plants.
Soil nutrients can reach plant roots by interception, mass flow, and diffusion. Interception occurs when roots have direct contact with a nutrient. However, interception only accounts for a small percentage of the total amount of nutrients taken up by plants.1
Mass flow, in which nutrients are moved toward a plant and into its roots with the water the plant is absorbing, accounts for a substantial amount of nutrient absorption. This process of absorbing nutrients is especially effective for mobile nutrients such as nitrate (NO3-), which move easily in soil water, though it is less effective at allowing plants to absorb immobile elements like phosphorus, which don’t move substantially in soil water. Specifically, mass flow has been found to account for about 80% of N movement into the root system of a plant, yet only 5% of the P movement.1
Diffusion accounts for the remainder of the soil nutrient absorption by plants. Diffusion is the chemical process by which substances move from areas of high concentration (the soil) to areas of low concentration (inside roots). Plants depend on diffusion to absorb immobile nutrients like P and K, primary macronutrients which they need in large amounts, but which are only available in low concentrations in soil water. Applying immobile primary macronutrients near plant roots can help plants absorb enough of these elements without relying on diffusion. Plants can often absorb enough of the immobile secondary macronutrients (i.e., Ca, Mg, S), because the solution concentrations of these elements are generally fairly high in soil relative to plant needs.1
How Nutrients Move Within a Plant
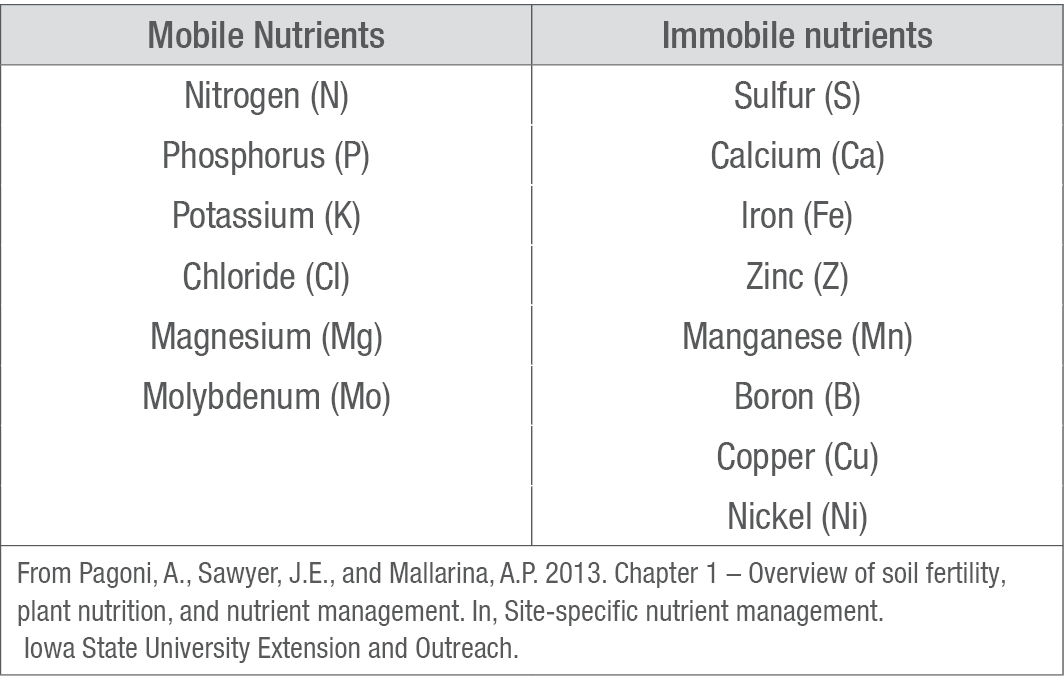
All nutrients move relatively easily from the roots to the growing portions of the plant, and some nutrients can also move from older, established tissue to newer, growing tissue if the plant is deficient in that nutrient. Knowing which nutrients are mobile within a plant can be useful in diagnosing plant nutrient deficiencies. If only the lower leaves are affected, then the plant is most likely deficient in a mobile nutrient. Conversely, if only the upper leaves show the deficiency, then the plant is likely deficient in an immobile nutrient which the plant cannot move from its older to newer leaves. Table 3 lists the six mobile and eight immobile mineral nutrients. Note that sulfur can be either mobile or immobile, depending on the plant’s degree of deficiency.1
Table 3. Mobile and Immobile Nutrients
The Influence of Soil pH on Nutrient Availability
Soil pH is a measure of acidity and alkalinity, terms used to describe the concentration of hydrogen ions (H+) in the soil solution.2 The pH scale is logarithmic, meaning each whole-number increase on the pH scale has 10 times as many hydrogen ions as the previous number.2 (For example, pH 8 has 10 times as many H+ ions as pH 7, and pH 8 has 100 times as many H+ ions as pH 6.) A pH of less than 7.0 is acidic, a pH of 7.0 is neutral, and a pH greater than 7.0 is alkaline.
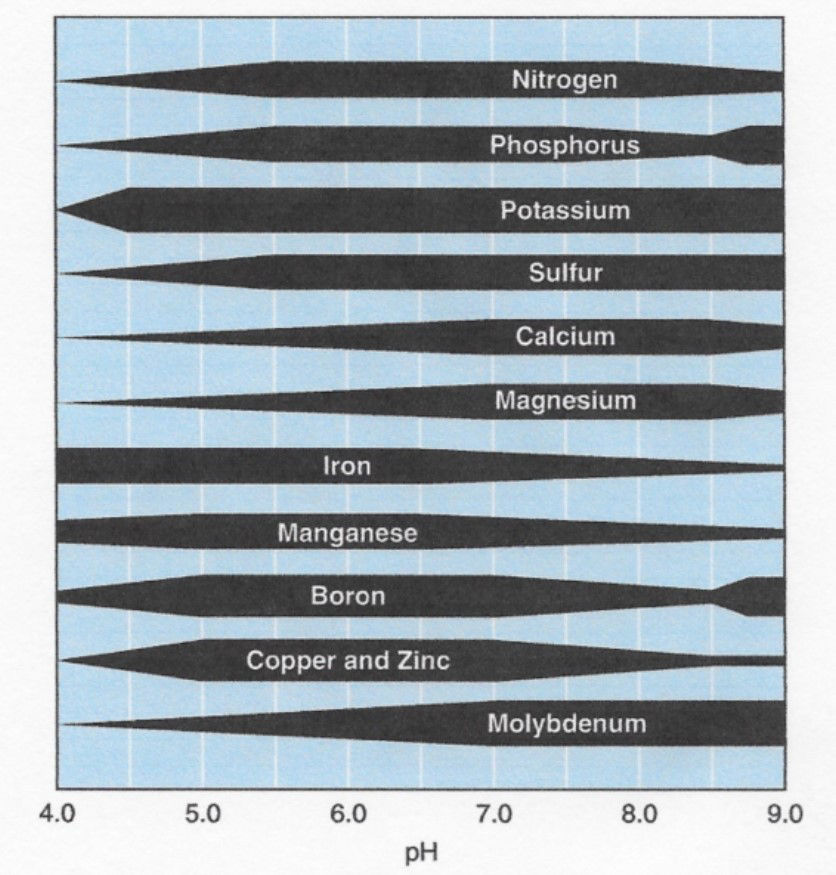
A plant’s ability to absorb nutrients is impacted by the soil pH, because different pH levels create different chemical environments that can make various elements more or less available for absorption (Figure 2).2 The primary macronutrients N, P, and K are at maximum availability when the soil pH is between 6.5 and 7.5, or slightly acidic to slightly alkaline. The elements Fe, Mn, B, Cu, and Zn becomes less available as soil pH increases above 7.0, while N, P, S, Ca, Mg, and Mo are less available in low pH environments (< 6.5).2 Given the differing availabilities of the essential macro- and micronutrients at different pH levels, a pH range of 6.0 to 7.0 is considered ideal for corn production.3,4
Low (too acidic) soil pH can cause symptoms similar to disease and chemical injury in corn. These symptoms include stunted plant development, uneven crop growth across a field, premature senescence of lower leaves, and interveinal chlorosis (yellowing between veins) on upper leaves. Acidic soil can also limit root growth and the activity of helpful soil microorganisms. Since these symptoms can mimic those of other causes, it is important to determine if soil pH is the cause of an abnormal plant appearance.
Low soil pH can usually be addressed with an appropriate lime application based on a soil test. These recommendations vary from state to state, so local university guidelines should be referenced. Liming rates are typically based on one or more factors, including:2,3
- Buffer pH provided on the soil test report.
- Cropping system.
- Soil type: Higher clay and organic matter content requires more lime.
- Depth of incorporation tillage (deeper tillage requires more lime).
- Limestone chemical composition (e.g., dolomitic lime or calcific lime).
- Limestone grind (finer limestone changes pH faster).
Photographs of Nutrient Deficiency Symptoms in Corn
The following pictures represent common nutrient deficiency symptoms in corn. When visual symptoms of nutrient deficiency are present—particularly symptoms of N and K deficiency—yield loss has already occurred.













Corn Nutrient Uptake and Partitioning
As new corn products are developed and grown at higher population densities than ever before, fertilization practices may need to be updated and finetuned to continue maximizing yield potentials.5 Two major aspects of plant nutrition are important to understand and manage for modern, high-yield corn production:
- Total nutrient uptake: The amount of a given mineral nutrient acquired during the growing season.
- Partitioning: The amount of each nutrient contained in each part of a plant. It is particularly important to consider the amount of each nutrient partitioned into corn grain, which will be removed from the field when the grain is harvested.
Plants have different nutrient needs at different times during their growth, which should be accounted for in fertilization practices.5 Some nutrients (e.g., N, K, Mg, Mn, and Fe), are primarily taken up by a plant during its vegetative growth stages. For example, it is critically important to supply enough N to meet corn’s peak needs of 7.8 lb N/day from the V10 to V14 growth stages. However, corn’s N uptake does not cease at VT/R1, since as much as 50 lb N/acre is accumulated and partitioned directly into the developing seeds during grain fill.5
Alternatively, corn accumulates P, S, Zn, and Cu during both vegetative and reproductive growth (ear development and grain-fill).5 A season-long supply of P, S, Zn, and Cu can help maximize the yield potential of corn products. Note that relative to total uptake, P is removed to a greater extent than any other nutrient. Agronomic practices which do not adequately replace removed P may lead to a depletion in soil fertility levels.5
Even though nutrient management is a complex process, improving understanding of uptake timing, uptake rates, partitioning, and the remobilization of nutrients by corn provides opportunities to optimize fertilizer rates and application timings.
Macronutrient Fertilization in Corn Production
Nitrogen (N)
Nitrogen (N) is required in the largest amounts and at the greatest cost.6 Corn grown for grain typically requires about 1.0 to 1.1 lb of N per bushel of yield goal, while corn grown for silage requires about 8 to 10 lb of N per ton of yield goal.7,8 Recommendations for N application rates can vary across regions, so growers should consult local university extension experts or a reputable soil testing laboratory.
Nitrogen must be provided by the soil or by fertilizer, since only one-third to two-thirds of the N added is recovered in the harvested corn.6 Nitrogen recovery is both variable and unpredictable—primarily due to the effects of weather on the release of soil N—and most of the unrecovered N is irreversibly lost from the soil through leaching. Additionally, biological and chemical reactions are continuously changing soil N from one form to another, and each form behaves differently in regard to its uptake by and availability to plants.6
Types of Nitrogen
Organic soil nitrogen is mostly insoluble and not directly available to plants. For example, soil that has three percent organic matter contains over 3,000 lb of organic N per acre, but only a small part—about one to five percent each growing season—is broken down to inorganic N available to plants. However, organic sources of N should not be ignored when estimating N fertilizer needs. Sods, legume cover crops, and animal manure can replace some or most of the inorganic N application.6
Inorganic ammonium (NH4+) is either released by the decomposition of organic matter or added as fertilizer. Ammonium is a relatively immobile ion that, unlike nitrate (NO3-), is not susceptible to leaching or denitrification, though under some conditions it may be lost as ammonia gas (NH3). Corn takes up NH4+ less readily than NO3-, but in most soils that are suitable for corn production, NH4+ is rapidly converted to NO3- in a process called ‘nitrification’. This reaction is completed within a few days to a month after fertilization, depending on soil temperatures.6
Nitrate, unlike ammonium, is a highly mobile ion that is readily available to plants. However, its high solubility in water and mobility within soil also make it susceptible to leaching, particularly on well-drained soils during long-lasting or very intense rainfall. Microbiological denitrification can also rapidly remove NO3- from soils, especially when soils are saturated. Some nitrate is lost almost every year in all soils, but such losses become serious when heavy rains fall within a month after fertilization. Soil drainage is the most reliable predictor of total nitrate loss, and tillage can also affect losses, so more N fertilizer is required in poorly drained soils and in no-till systems.6
Note that while plants generally respond equally to all inorganic forms of N fertilizer, urea (CH4N2O) can be an important exception. Urea is rapidly converted to ammonium (NH4+) in the soil, making the soil near the fertilizer granule more alkaline and favoring the formation of NH3 gas. This means that a large fraction of N may be volatilized and lost as NH3 if urea is broadcast on moist, warm soil. Nitrogen losses can be minimized by avoiding these conditions or by incorporating the urea into the soil. If urea is available at a lower price than alternative N forms, it may be more economical to use—despite the greater risk of loss. Urea is not a slow-release fertilizer.6
Nitrogen Application Methods
Nitrogen can be applied as a liquid or a solid, and neither is more effective than the other. When choosing between liquid and solid N, consider the cost, handling, and convenience of the application method.6
As previously mentioned, plants need different nutrients in different amounts throughout their development. Young corn plants (4 to 6 weeks) require very little N, and most of that can be supplied by the soil, so a split or delayed nitrogen application timing can increase the efficiency of N use in corn.6 Additionally, because soil is typically wetter and more likely to lose N in early spring, delayed applications are most beneficial where denitrification and leaching losses are greatest, particularly on poorly or moderately well-drained soils. As a general guideline for these types of soils, if two-thirds or more of the N is applied 4 to 6 weeks after planting, the total N application can be reduced by 25 to 50 pounds per acre. However, note that fall N application is generally not recommended. In areas where fall anhydrous ammonia can be applied, soil temperature should be below 50 °F before application begins.6
The placement of nitrogen fertilizer has not been found to have a consistent effect on the efficiency of N fertilizers containing NH4+ and NO3- salts.6 Researchers have sometimes observed benefits from banding or sub-surface application under certain conditions; however, the results do not justify the cost of machinery alterations or the extra time that is usually required to make those applications. The risk of immediate volatilization after applying urea (CH4N2O) can be reduced if it is incorporated into the soil with tillage. Rainfall, or an irrigation application of at least 0.25 inches of water, would also be sufficient to incorporate urea and reduce the chances of volatilization.6
Nitrogen inhibitors are chemicals that act specifically to inhibit the conversion of NH4+ to NO3- and indirectly reduce the chance of early-season leaching and denitrification. These chemicals are beneficial only when such losses are potentially substantial. In many areas where corn is produced, plants have responded positively to the use of inhibitors on moderately well-drained, no-till soils.6
Phosphorus (P) and Potassium (K)
Both phosphorus (P) and potassium (K) are required in large quantities for good corn growth and yields, and it is especially important that plants receive enough P2O5 and K2O during the first half of the growing season. By the time kernels start filling (70 to 75 days after seedling emergence and 10 to 15 days after silking) the plant should have taken up about 70% of its P2O5 requirement and nearly 90% of its K2O requirement.6 Corn grain removes about 0.32 to 0.38 lb of P2O5, and 0.22 to 0.27 lb of K2O per bushel; therefore, the grain produced by a 200 bu/acre corn crop removes about 65 to 75 lb of P2O5 and 45 to 55 lb of K2O per acre.7,9 Approximately three-fourths of the P2O5 and about one-third of the K2O taken up by the crop is contained in the grain, while the remainder is allocated to the leaves, stalk, roots, husks, and cob.5 In silage production, all P2O5 and K2O taken up by the plant, except that in the roots and stubble, is removed from the soil. Nutrient removal rates of corn silage are estimated to be about 3.5 lb of P2O5, and 9.0 lb of K2O per ton (measured at 65% moisture content).8
Both P and K are considered immobile elements, because they react with the soil in many ways that minimize their movement with soil water, and environmental conditions have strong effects on their availability to plants.6 This is particularly true of P, since it forms compounds with soil Ca, Fe, aluminum (Al), Mn, and Zn which are less soluble than the P compounds in fertilizer. A soil pH between 6.0 and 6.5 can help plants properly absorb P applications, as at this pH range much of the fertilizer P will react to form monocalcium or dicalcium phosphates—types of P that are more soluble than the Fe, Al, and Mn phosphate that form at lower pH levels. Therefore, good liming practices may increase the availability of P to plants.6
Loss of P and K through topsoil erosion is a concern in many areas of the Corn Belt.6 Potassium, similar to NH4+, is retained on clays and organic matter by cation exchange (CEC). The CEC of most soils, except for those with high sand content, is generally high enough to hold an adequate reservoir of readily available K+, though these can be lost if the soils containing them are eroded. Conversely, leaching of P and K is generally limited to low-CEC soils.6
Using a reliable soil testing lab is the best way to determine soil content of plant-available P and K. Table 4 contains recommendations made by university soil labs and many independent labs, based on the Mehelich III extraction (see example Table 4).6 Check with your local extension service or fertilizer dealer for a specific recommendation for your area.
Table 4. Recommended Rates of Phosphate and Potash Application (lb/acre) for Corn, as Related to Soil Test Value.
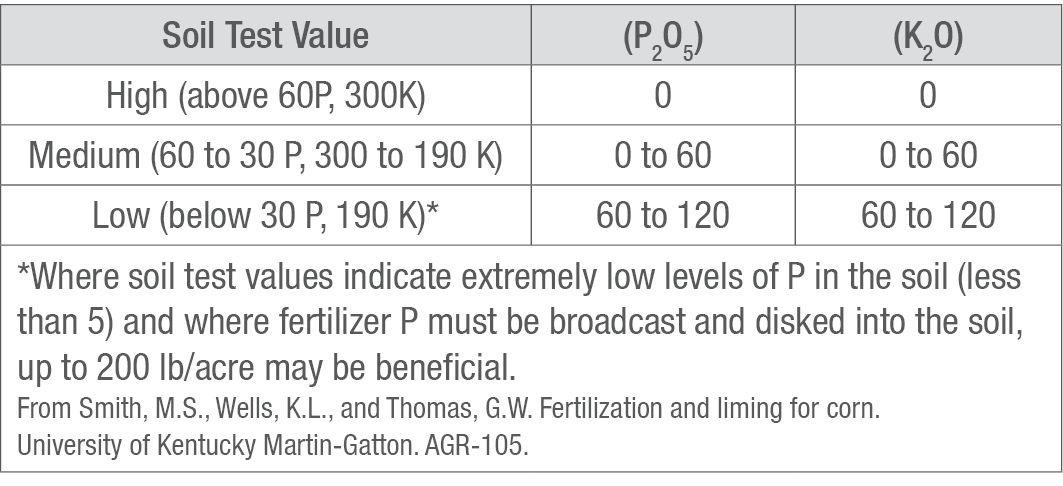
Types of Phosphorus and Potassium
Commercial fertilizers are the most widely used sources of P and K for corn production, including triple superphosphate (0-46-0), diammonium phosphate (18-46-0), monoammonium phosphate (11-52-0), and a wide array of other liquid and dry ammoniated phosphates. Murate of potash (0-0-60) is by far the most common K fertilizer for corn. Other K sources include sulfate of potash (0-0-50) and sulfate of potash magnesia (0-0-18, 11S, 18Mg). All common sources of P and K fertilizer are considered equally effective for corn when used at the recommended rates and applied properly. Solid and liquid forms of P are also considered equally effective.6
Organic sources of P and K, such as animal manures and sewage sludge, may also be used for supplying P and K. The nutrient content of manure and sewage sludge varies, so a nutrient analysis of the manure and/or sludge is necessary to determine how valuable it will be as a soil amendment. Additionally, if manure or sewage is used, the heavy metals content (nickel, cadmium, and chromium) should be checked to prevent toxic build-up in the soil.6
Phosphorus and Potassium Application Methods
Placement of P and K by broadcast is the most convenient method of application, although large amounts are required at very low soil test levels.6 Banded applications (2 inches to the side and 2 inches below the seed) can increase agronomic efficiency of P and K, making it possible to decrease the usual rate by 1/3 to 1/2. A starter effect (improved initial growth) is likely to result from a banded application. This may appear substantial during the early growing season, but rarely does it increase yields, provided the P and K are used at the recommended rates.6
Sources
1Pagoni, A., Sawyer, J.E., and Mallarina, A.P. 2013. Chapter 1 – Overview of soil fertility, plant nutrition, and nutrient management. In, Site-specific nutrient management. Iowa State University Extension and Outreach. https://www.agronext.iastate.edu/soilfertility/sitespecific4R.html
2Fernández, F.G. and Hoeft, R.G. Managing soil pH and crop nutrients. In, Illinois agronomy handbook. https://extension.cropsciences.illinois.edu/handbook/pdfs/chapter08.pdf
3Culman, S., Fulford, A., Camberato, J., and Steinke, K. 2020. Tri-state fertilizer recommendations for corn, soybean, wheat, and alfalfa. Ohio State University Extension. Bulletin 974. https://extensionpubs.osu.edu/tri-state-fertilizer-recommendations-for-corn-soybean-wheat-and-alfalfa-pdf/
4Miller, J. 2016. Soil pH affects nutrient availability. University of Maryland Extension. FS-1054. https://extension.umd.edu/sites/extension.umd.edu/files/publications/FS-1054%20Soil%20pH%20and%20Nutrient%20Availbility_Update_12_2021.pdf
5Crop nutrient uptake and partitioning. University of Illinois Urbana-Champaign. https://cropphysiology.cropsci.illinois.edu/nutrient-uptake-and-partitioning/
6Smith, M.S., Wells, K.L., and Thomas, G.W. Fertilization and liming for corn. University of Kentucky Martin-Gatton. AGR-105. https://www2.ca.uky.edu/agcomm/pubs/agr/agr105/agr105.htm
7Gerwing, J. and Gelderman, R. 2005. Fertilizer recommendations guide. South Dakota State University. https://extension.sdstate.edu/sites/default/files/2023-06/P-00039-2023-v2.pdf
8Ward, R.C. 2020. Ward guide. Ward Laboratories. https://www.wardlab.com/resources/ward-guide/
9Mallarino, A.P., Sawyer, J.E., Barnhart, S.K., and Licht, M.A. 2023. A general guide for crop nutrient and limestone recommendations in Iowa. Iowa State University Extension and Outreach. PM-1688. https://store.extension.iastate.edu/product/5232
Web sources verified 07/26/24. 1213_70649