Managing Blackleg in Canola
October 25, 2022
Blackleg (Leptosphaeria maculans) is a stubble-borne fungus that infects canola crops across the prairies. Plants can be infected throughout the growing season with seedling infection being the most damaging. Though this disease is favoured by warm, moist conditions, it can also be found in dry years or regions. The adaptability of blackleg has led to disease prevalence across the canola growing region year after year. Because of the prevalence and severity of the disease, much effort has been put into plant breeding.1
Blackleg is one of the most serious diseases affecting canola production in North America.2 The first resistant canola varieties were sown in the early 1990’s. Since then, many canola varieties have been developed with resistance to blackleg. Recently, breakdown of the current resistance has been observed.1,2
- Annual disease surveys have shown that blackleg is found in most canola fields in both Canada and the United States in over 50 percent of fields each year.2,3,4,5
- Short crop rotations and a warm, wet environment can result in areas with higher disease severity. Blackleg can be severe even in dry years if rain showers are timely to disperse spores, and plants become infected at early stages.
- When severe infection occurs, yield loss can be up to 50%.1
Disease Cycle
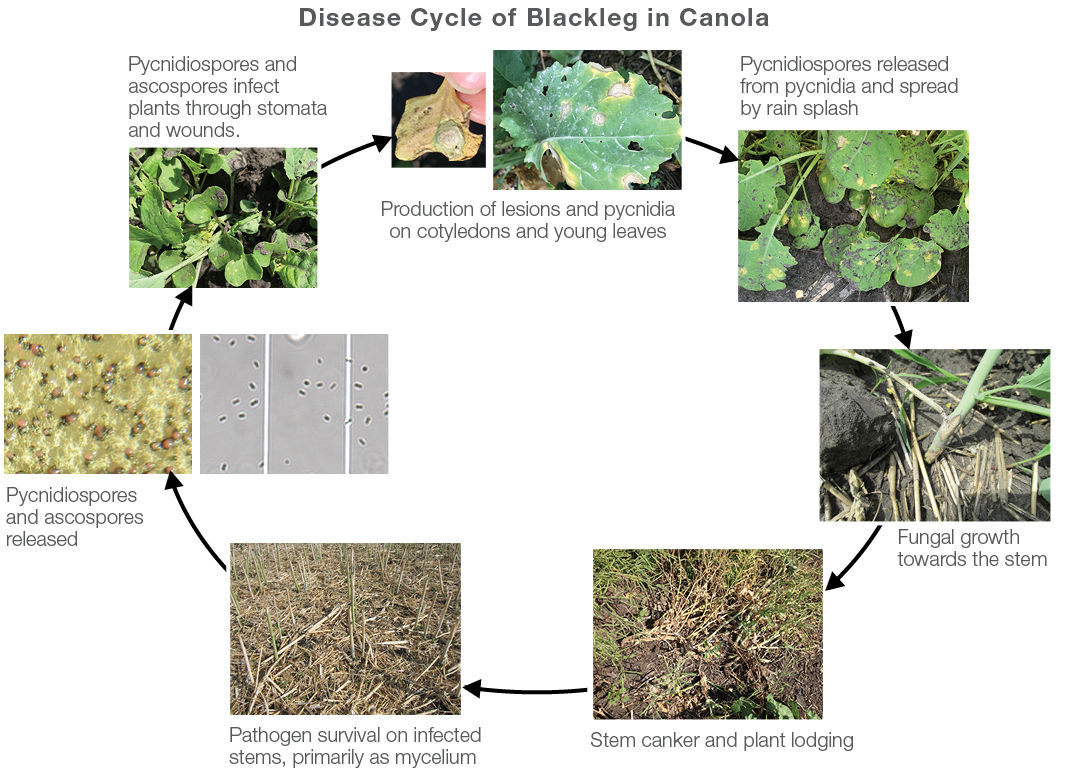
Figure 1. Disease cycle of blackleg (L. maculans) on canola. Photos courtesy of Xuehua Zhang.
Blackleg overwinters on infected canola or mustard residue. Spores are released in the spring and move with wind and rain. Spores can cause infection in canola at any crop stage. Yield loss is greatest when blackleg infects in the early growing stages prior to the six-leaf stage.2,5 Spores infect the canola at the leaf and the disease spreads through the plant down the stem. Finally, the stem is girdled at the base of the plant (Figure 1).1 The amount of girdling can indicate potential yield loss.6
Disease Identification

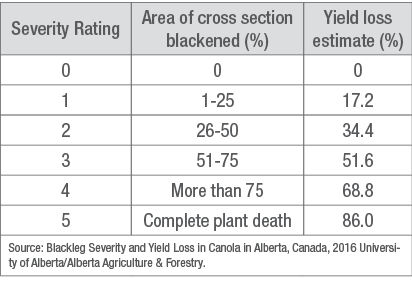
Initial infection appears as a white lesion dotted with black pycnidia anywhere on the plant. The best way to identify blackleg is by cutting the stem in a cross section just below the base of the stem at swath timing. Systemic blackleg appears as blackened sections in the stem cross-section (Figure 3). Blackleg is assessed in terms of incidence and severity. Incidence is the percentage of plants infected per field. Severity is a rating on a zero to five scale of the basal stem cross-section with zero being no infection and 5 being 100% infected and dead. Yield loss for a plant can be estimated by the severity rating (Table 1).
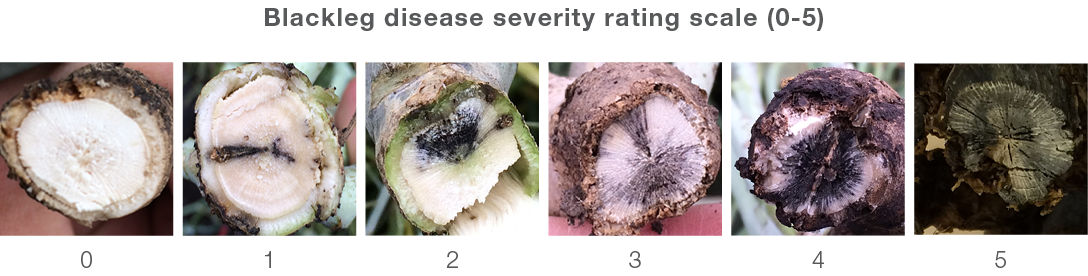
Table 1. Yield loss estimate by severity rating.
Sample 50 to 100 plants which are representative of the field. To calculate an estimate of field disease severity, assess each plant collected based on the disease rating scale (Figure 3), add up the total, and divide by the number of plants assessed to find the average for the field.
Recent Plant Breeding Efforts
There are two types of resistance that work in the canola plant against blackleg infection: minor gene resistance and major gene resistance.
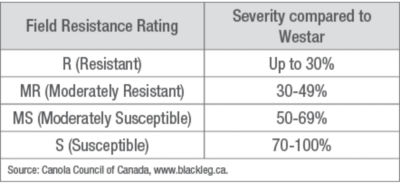
Minor gene resistance works against all blackleg strains by slowing the infection in the plant. This involves a combination of many genes in the plant working together against a blackleg infection. The limitation is that minor gene resistance does not provide complete control of blackleg. Minor gene resistance is accounted for in the Field Resistance Rating. Prior to registration, new canola varieties are grown in trials and compared to a highly susceptible variety (Westar) (Table 2). The ratings are as follows:
Table 2. Field Resistance Rating compared to highly susceptible Westar variety.
Major gene resistance prevents infection of the plant by the specific blackleg race to which it is targeted. The resistance genes in the plant must match the infecting genes in the pathogen for plant protection against blackleg. A breakdown occurs when a variety rated “R” has unexpectedly high levels of infection. This is likely due to a shift in blackleg population in the field so that the resistance genes are no longer matched and blackleg is able to infect the plant. This leads to infected plants relying on minor gene resistance to prevent the spread of infection.
Management
Crop rotation. The spores live on the stubble and once the stubble has broken down, many of the spores die. The recommended rotation away from canola is two years (and up to three years in areas of high infection). Make sure to control volunteer canola and mustard weeds in the non-canola crop to prevent blackleg from reproducing on these plants.1,2 A good crop rotation is especially important in regions which favour the disease with warm, moist, and humid climates.
Scouting. Scout at swathing or at harvest if straight-cutting. This is the time when the disease is easiest to identify by cutting the stems at the base of the root and evaluating the stem girdling. It is recommended to sample at least 50 plants while moving in a “w” pattern through the field.1
Lab testing. The mixture of races that can occur is very dynamic and can shift quickly; therefore, knowing which race is in the field will help match up canola products with the best resistance gene package. To help match the major gene resistance to the blackleg races in the field, infected canola stems selected from the field can be sent to a lab for analysis. Swath-timing is the best time to collect stem sections, but stubble can be collected prior to planting as well. The phenotype that is reported can help guide the seed selection process. Match the avirulence genes (eg. AvrLm2) found in the majority of the blackleg races in the field to the resistance gene in the canola variety (Rlm2 which is found in Resistance group B). In this example, growing seed which is labelled as Resistance group B matches the blackleg races found in the field and could provide better success with major gene resistance. There are private laboratories available in the U.S. to determine the race of blackleg.
Variety Selection.
- Choose a canola variety with a field resistance rating “R” for “Resistant”.
- If resistance of the plant has been overcome by blackleg in previous years and major gene resistance is unknown, then use a different variety with known major gene resistance and consider testing the field for blackleg races at swathing.
- Consider matching the major gene resistance of the canola seed to the blackleg strains in the field. Testing infected stubble from the previous canola crop can help determine blackleg strains in each field.
Fungicides.
There are fungicides available for application to canola for blackleg control, so consult your local extension office to determine what products are registered for your area, as some states may have local labels. While there are fungicides, one study indicted that application of fungicides was only beneficial when applied to susceptible canola products under high disease pressure.7
Contact your local Bayer representative for help managing blackleg on your farm.
Sources
1 Canola Council of Canada. 2020. Blackleg. Canola Encyclopedia. https://www.canolacouncil.org/canola-encyclopedia/diseases/blackleg/.
2 Shahoveisi, F. Markell, S. Luis, del Río Mendoza, E. 2020. Canola Diseases: Blackleg. North Dakota State University Extension. https://www.ag.ndsu.edu/publications/crops/canola-diseases-blackleg.
3 Harding, M.W., Daniels, G.C., Burke, D.A., Pugh, C.A., Hill, T.B., Zahr, K., Sarkes, A., and Feng, J. 2019. A survey for blackleg and sclerotinia stem rot on canola in Alberta in 2019. Canadian Plant Disease Survey 2020 Volume 100: Disease Highlights 2019, Canadian Journal of Plant Pathology, 42:sup1, 1-175, DOI: Canadian Plant Disease Survey 2020 Volume 100: Disease Highlights 2019.
4 McLaren, D.L., Kaminski, D., Graham, J., Bargen, E., Buss, T., Clouson, N., Cummer, T., Farooq, A., et al. 2019. Survey of canola diseases in Manitoba in 2019. In Canadian Plant Disease Survey 2020 Volume 100: Disease Highlights 2019. Canadian Journal of Plant Pathology. 42:sup1, 1-175.
5 Markell, S., del Rio, L. Halley, S., Mazurek, Shanna, Mathew, F. and Lamey, A. 2008. Blackleg of Canola. North Dakota State University Extension.
6 Hwang, S., Strelkov, S.E., Peng, G., Ahmed, H., Zhou, Q., and Turnbull, G. 2016. Blackleg (Leptosphaeria maculans) Severity and yield loss in canola in Alberta, Canada. Plants. pg. 7. Government of Alberta. Blackleg of canola - pest. https://www.alberta.ca/blackleg-of-canola-pest.aspx.
7 Peng, G. et al. 2020. Early fungicide treatment reduces blackleg on canola but yield benefit is realized only on susceptible cultivars under high disease pressure. Canadian Journal of Plant Pathology. https://www.tandfonline.com/doi/abs/10.1080/07060661.2020.1824166?journalCode=tcjp20.
Web sources verified 10/4/2022.
2011_81059